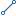Business Rule Event Sk
Business Rule Event Sk Drug Administration Ts
Drug Administration Ts Drug Equivalent Nm
Drug Equivalent Nm Drug Group Nm
Drug Group Nm Drug Nm
Drug Nm Drug Order Discontinue Ts
Drug Order Discontinue Ts Drug Order End Ts
Drug Order End Ts Drug Order Start Ts
Drug Order Start Ts Effective From Dt
Effective From Dt Effective To Dt
Effective To Dt Load Info Sk
Load Info Sk Source Code Sk
Source Code Sk Standard Drug Code Sk
Standard Drug Code Sk Standard Drug Dosage Code Sk
Standard Drug Dosage Code Sk Standard Drug Group Code Sk
Standard Drug Group Code Sk Standard Drug Route Code Sk
Standard Drug Route Code Sk Standard Drug Schedule Code Sk
Standard Drug Schedule Code Sk Standard Drug Susceptibility Code Sk
Standard Drug Susceptibility Code Sk Standard Drug Unit Of Measure Code Sk
Standard Drug Unit Of Measure Code Sk Standard Specimen Source Code Sk
Standard Specimen Source Code Sk Tenant Sk
Tenant Sk Valid From Ts
Valid From Ts Valid To Ts
Valid To Ts