 Effective From Dt
Effective From Dt Effective To Dt
Effective To Dt Experimental Unit Sk
Experimental Unit Sk Identification Num
Identification Num Load Info Sk
Load Info Sk Source Code Sk
Source Code Sk Status Code Sk
Status Code Sk Status Dt
Status Dt Subgroup Code Sk
Subgroup Code Sk Tenant Sk
Tenant Sk Valid From Ts
Valid From Ts Valid To Ts
Valid To Ts
| Atomic Warehouse Model Data Model |
| Description | A physical entity that is the primary unit of interest in a specific research objective. For example: If all pigs in a pen receive the same intervention in their feed, and the primary observations and analyses of interest are associated with the entire pen (for example, total feed consumed, total weight of all pigs combined), then the pen of pigs rather than the individual animal is the experimental unit. [example from the CDISC/HL7 Study Participation message] A human may have 10 patches of skin each considered an experimental unit. A product may have 10 bearings in it, each considered an experimental unit. Alternatively, the whole human or product may be an experimental unit. Depending on the research objectives, a single study may have multiple levels of experimental units, such as whole people and patches of skin. In an interventional study, the experimental unit is assigned to an intervention. The experimental unit is also the unit of primary statistical analysis. Commonly the individual study subject (animal, person or product) is the experimental unit. Different experimental units must be capable of receiving different experimental interventions. |
| Attributes | |
 Effective From Dt Effective From Dt |
|
 Effective To Dt Effective To Dt |
|
 Experimental Unit Sk Experimental Unit Sk |
|
 Identification Num Identification Num |
|
 Load Info Sk Load Info Sk |
|
 Source Code Sk Source Code Sk |
|
 Status Code Sk Status Code Sk |
|
 Status Dt Status Dt |
|
 Subgroup Code Sk Subgroup Code Sk |
|
 Tenant Sk Tenant Sk |
|
 Valid From Ts Valid From Ts |
|
 Valid To Ts Valid To Ts |
|
| Relationship | |
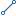 Experimental Unit Detail_Experimental Unit_FK Experimental Unit Detail_Experimental Unit_FK |
|
| Primary Key | |
 Experimental Unit Detail PK Experimental Unit Detail PK |
|
| Dependencies | |
 |
|
| Reverse Dependencies | |
 |
|
| Attribute Details |
 Effective From Dt
Effective From Dt
| Description | Establishes a period where a set of attributes are true according to the business. |
| Data Type | Standards - Data Domains.ddm/Data Domains/Date [DATE] |
| Is Part Of PrimaryKey | false |
| Is Required | true |
| Is Derived | false |
| Is Surrogate Key | false |
 Effective To Dt
Effective To Dt
| Description | Ends a period of effectivity. |
| Data Type | Standards - Data Domains.ddm/Data Domains/Date [DATE] |
| Is Part Of PrimaryKey | false |
| Is Required | false |
| Is Derived | false |
| Is Surrogate Key | false |
 Experimental Unit Sk
Experimental Unit Sk
| Description | Surrogate key for the anchor entity. |
| Data Type | Standards - Data Domains.ddm/Data Domains/Surrogate Key Large [LONG] |
| Is Part Of PrimaryKey | true |
| Is Required | true |
| Is Derived | false |
| Is Surrogate Key | false |
 Identification Num
Identification Num
| Description | A unique symbol that establishes identity of the experimental unit. For example: Patient number 7 on a study |
| Data Type | Standards - Data Domains.ddm/Data Domains/Alphanumeric [VARCHAR(80)] |
| Is Part Of PrimaryKey | false |
| Is Required | false |
| Is Derived | false |
| Is Surrogate Key | false |
 Load Info Sk
Load Info Sk
| Description | The surrogate key of the load information entry describing the details regarding the loading of the row. |
| Data Type | Standards - Data Domains.ddm/Data Domains/Surrogate Key Large [LONG] |
| Is Part Of PrimaryKey | false |
| Is Required | true |
| Is Derived | false |
| Is Surrogate Key | false |
 Source Code Sk
Source Code Sk
| Description | The origin of the data identifying the actual load source, vendor, manual key entry, or context of the data in a specific row in the database. |
| Data Type | Standards - Data Domains.ddm/Data Domains/Surrogate Key [INTEGER] |
| Is Part Of PrimaryKey | false |
| Is Required | true |
| Is Derived | false |
| Is Surrogate Key | false |
 Status Code Sk
Status Code Sk
| Description | Identifies the phase in the lifecycle of the experimental unit. For example: Active Cancelled Pending Suspended Terminated Nullified |
| Data Type | Standards - Data Domains.ddm/Data Domains/Surrogate Key [INTEGER] |
| Is Part Of PrimaryKey | false |
| Is Required | false |
| Is Derived | false |
| Is Surrogate Key | false |
 Status Dt
Status Dt
| Description | The date and time the status for an experimental unit as part of a study or clinical trial is defined. |
| Data Type | Standards - Data Domains.ddm/Data Domains/Date Time [TIMESTAMP] |
| Is Part Of PrimaryKey | false |
| Is Required | false |
| Is Derived | false |
| Is Surrogate Key | false |
 Subgroup Code Sk
Subgroup Code Sk
| Description | Specifies the identification of uniform groups of experimental units for separate analysis or treatment. For example: Clinical Data Update System (CDUS) Reporting (for the National Cancer Institute (NCI) |
| Data Type | Standards - Data Domains.ddm/Data Domains/Surrogate Key [INTEGER] |
| Is Part Of PrimaryKey | false |
| Is Required | false |
| Is Derived | false |
| Is Surrogate Key | false |
 Tenant Sk
Tenant Sk
| Description | The surrogate key of the entry identifying the legal owner of the data. |
| Data Type | Standards - Data Domains.ddm/Data Domains/Surrogate Key [INTEGER] |
| Is Part Of PrimaryKey | false |
| Is Required | true |
| Is Derived | false |
| Is Surrogate Key | false |
 Valid From Ts
Valid From Ts
| Description | Establishes a period where a set of attributes are true in the source system. This would be populated with the transaction timestamp and would be used for the snapshot date. |
| Data Type | Standards - Data Domains.ddm/Data Domains/Timestamp [TIMESTAMP] |
| Is Part Of PrimaryKey | true |
| Is Required | true |
| Is Derived | false |
| Is Surrogate Key | false |
 Valid To Ts
Valid To Ts
| Description | Ends a period of validity. |
| Data Type | Standards - Data Domains.ddm/Data Domains/Timestamp [TIMESTAMP] |
| Is Part Of PrimaryKey | false |
| Is Required | false |
| Is Derived | false |
| Is Surrogate Key | false |
| Relationship Details |
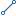 Experimental Unit Detail_Experimental Unit_FK
Experimental Unit Detail_Experimental Unit_FK
| Is Identifying Relationship | true |
| Child Table | Experimental Unit Detail |
| Child Multiplicity | ZERO_TO_MANY |
| Child Referential Integrity: On Delete | NONE |
| Child Referential Integrity: On Insert | NONE |
| Child Referential Integrity: On Update | NONE |
| Parent Table | Experimental Unit |
| Parent Multiplicity | ONE |
| Parent Referential Integrity: On Delete | NONE |
| Parent Referential Integrity: On Insert | NONE |
| Parent Referential Integrity: On Update | NONE |
| Primary Key Details |
 Experimental Unit Detail PK
Experimental Unit Detail PK
| Key Attribute | Experimental Unit Sk |
| Key Attribute | Valid From Ts |
| Atomic Warehouse Model Data Model |